Previously:
Disclaimer: I am not a medical professional and this is not medical advice.
 Since 2018, I’ve been covering emerging findings on the relationship between the progestin cyproterone acetate (CPA), commonly used outside of the United States as a testosterone blocker for trans women, and the risk of developing a type of large benign brain tumor known as a meningioma. Meningiomas frequently have progesterone receptors, and many are small and stable or very slow-growing without causing any symptoms, but increased action at its progesterone receptors can stimulate it to grow and cause symptoms due to its volume. This stimulation can be the result of normal physical progesterone levels – cis women are much more likely than cis men to experience meningioma, and pregnancy can accelerate the growth of meningioma – or it can be due to prolonged use of strong synthetic progestins such as CPA and certain other progestins appearing in hormone replacement therapy or contraception for cis women.
Since 2018, I’ve been covering emerging findings on the relationship between the progestin cyproterone acetate (CPA), commonly used outside of the United States as a testosterone blocker for trans women, and the risk of developing a type of large benign brain tumor known as a meningioma. Meningiomas frequently have progesterone receptors, and many are small and stable or very slow-growing without causing any symptoms, but increased action at its progesterone receptors can stimulate it to grow and cause symptoms due to its volume. This stimulation can be the result of normal physical progesterone levels – cis women are much more likely than cis men to experience meningioma, and pregnancy can accelerate the growth of meningioma – or it can be due to prolonged use of strong synthetic progestins such as CPA and certain other progestins appearing in hormone replacement therapy or contraception for cis women.
The likelihood of developing meningioma related to progestin use is known to increase substantially as higher doses of progestins are taken for longer periods of time, and trans women are likely to take high doses of CPA for many years as part of a long-term HRT regimen. In one sample, trans women were found to be four times more likely than cis women to develop meningioma, and all of these trans women had been using CPA (Nota et al., 2018). The link between CPA dosage and meningioma risk was more recently demonstrated in a population-wide study in France of all cis women and trans women who had taken CPA (Weill et al., 2021). Compared to a control group those who had taken a cumulative dosage of less than 3,000mg total of CPA, those who had taken 12,000-36,000mg were 6.4 times as likely to experience a meningioma requiring surgery or radiation treatment, and those who had taken over 60,000mg were 21.7 times as likely to develop meningioma. This risk is partially reversible: when CPA was stopped for at least one year, the risk of meningioma declined and was now only 1.8 times that of the control group.
Cumulative dosage is a function of daily dosage and overall days using CPA; trans women may take testosterone blockers such as CPA for many years until genital surgery, and some may not have genital surgery at all, continuing to take CPA indefinitely. Overall time spent on CPA will tend to be very high – and the daily dosage for trans women is a subject of contention. The appropriate dosage for use as a testosterone blocker in trans women was noted to be as high as 100mg daily in the 2009 Endocrine Society guidelines (Hembree et al., 2009) and as high as 50mg in the updated 2017 guidelines (Hembree et al., 2017), while recent clinical studies have indicated a dose of 10mg daily blocks testosterone to the same extent as higher doses (Kuijpers et al., 2021, Meyer et al., 2020). Trans women may have been taking more CPA than was actually necessary by a factor of 10. Their risk of meningioma may have grown by a factor of 10 as well.
Mikkelsen et al. (2021) have now provided confirmation of this dosage-associated risk in a similar study spanning the health records of the population of Denmark, including prescribed doses of CPA and any later treatment for meningioma. Compared to those who had never taken CPA, those who had taken up to 10,000mg of CPA were 7 times as likely to have developed meningioma, and those who had taken over 10,000mg were 19.2 times as likely. The risk of meningioma among those who had used CPA but stopped was lower – 5.4 times as likely as those who never took CPA.
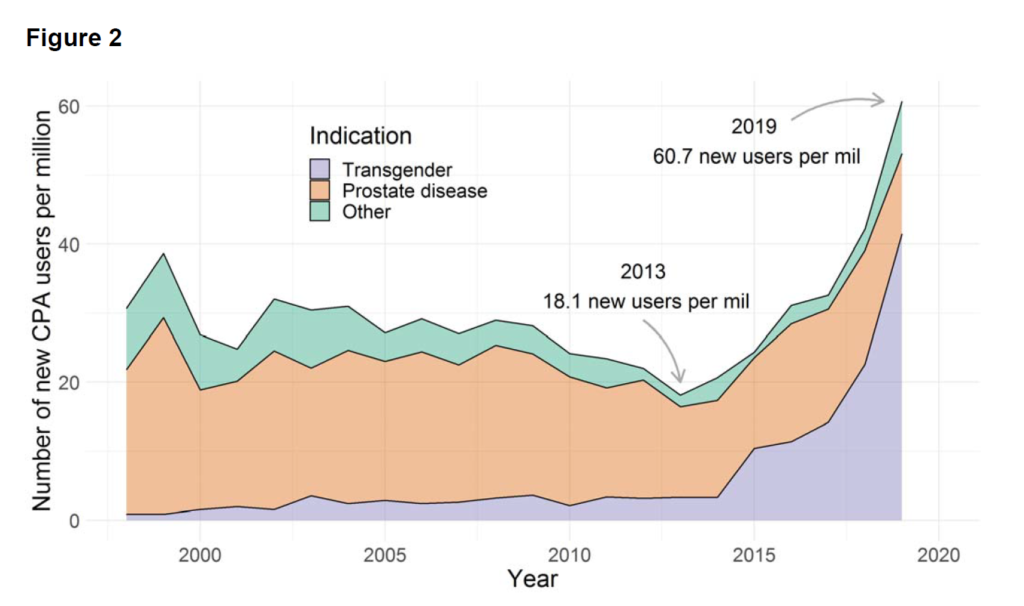
Notably, the authors found that in Denmark the overall usage of CPA had tripled from 2013 to 2019 and that this growth was largely due to increased use of feminizing HRT: “From 2013 to 2019 the number of new users increased from 18.1 to 62.3 new users per million, while the proportion of new users who were transgender increased from 18.4 to 68.3%.”
Trans women may disproportionately bear the burden of CPA-associated meningioma. Even at the lower dosage of 10mg, a trans woman who uses CPA for 10 years – a period of time which isn’t at all unusual for trans women who haven’t had genital surgery – will have taken 36,500mg in total, while the long-used higher dosage of 100mg would produce a cumulative exposure of 365,000mg over a decade. Weill et al. pointed out how quickly meningiomas occurred in trans women in the French population when high-dose CPA was used:
The three people with meningioma in the exposed group who required surgery were those taking very high daily doses of 100-150 mg for relatively short exposure periods of 3-4.5 years.
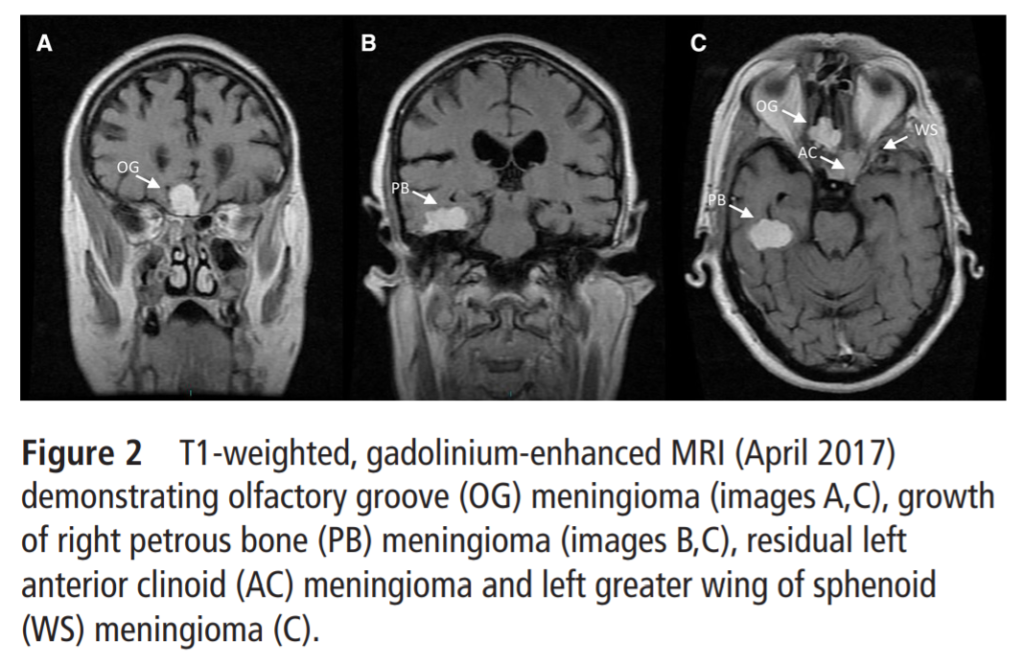
The dramatic effect of high-dose CPA on meningioma growth was recently illustrated in a case report on a 69-year-old trans woman who had reportedly taken 200mg CPA daily from 1998 to 2018, which was much higher than any historically recommended dose (Millward et al., 2021). Meningiomas were first detected in 2013 after she began experiencing tremors. These tumors were monitored as growing extensively from April 2017 to July 2018 as depicted below, at which time she was experiencing “significant deterioration in cognition, memory and balance” and was taken off CPA:
Discontinuing CPA can lower the risk of developing meningioma going forward, but in those who already have a CPA-associated meningioma, this can also directly reduce the volume of these tumors over time. Voormolen et al. (2021) tracked the MRI readings over time of 108 cis women with a total of 262 CPA-associated meningiomas, finding that these tumors grew during CPA usage and shrank when CPA was stopped: “Meningiomas grew (average velocity of 0.25 mm3/day) while patients were using PCA and shrank (average velocity of −0.54 mm3/day) after discontinuation of PCA.” 71% of these meningiomas decreased in size when CPA was stopped, and another 20% stabilized and stopped growing.
Using the lowest effective dosage of CPA is one way for trans women taking HRT to minimize their risk of meningioma. Alternately, other antiandrogens may be used that do not present this risk. As Millward et al. point out:
Multiple options are available, such as goserelin acetate or leuprolide, which are GnRH agonists that effectively reduce testosterone levels, spironolactone, which directly blocks androgens during their interaction with the androgen receptor, and may also have estrogenic activity, and CPA among other progestins, such as nomegestrol acetate and chlormadinone acetate.
Medroxyprogesterone acetate (MPA) is another synthetic progestin that does not appear to be linked to meningioma growth, and has been used as part of feminizing HRT as an antiandrogen (Jain et al., 2019). Evidence of the risks associated with long-term use of CPA is continuing to accumulate, and trans women are highly exposed to those risks. It is crucial for trans women to take this information into account as they make decisions about their medical transition. ■